


-
Argentina (Español)

-
Australia (English)

-
Bahrain (English)

-
Chile (Español)

-
Deutschland (Deutsch)

-
Europe (English)

-
France (Français)

-
Greece (Ελληνικά)

-
Italia (Italiano)

-
日本 (日本語)

-
대한민국 (한국어)

-
Kuwait (English)

-
Oman (English)

-
Polska (Polskie)

-
Qatar (English)

-
Saudi Arabia (English)

-
Spain (Español)

-
Sverige (Svenska)

-
台灣 (中文)

-
United States (English)

-
UAE (English)

Assessment of motor function in spinal muscular atrophy (SMA)
The natural progression of SMA can be assessed by age- and ability-
appropriate motor function scales and electrophysiological
measurement of motor-unit health1,2
Motor function scales
A number of motor function scales* have been developed that have proven useful in a range of settings, including:2-4
- Assessment of the natural history of SMA in clinical studies
- Establishment of a baseline in order to observe potential functional motor benefits of investigational therapeutic agents in clinical trials
*Below is not a comprehensive list of motor function scales.
Hammersmith Infant Neurological Examination (HINE)
Designed to be a simple and scorable method for evaluating infants from 2 to 24 months, the HINE includes 3 sections containing 26 items that assess different aspects of neurologic function:5,6
- Section 1: Neurologic examination assessing cranial nerve function, posture, movements, tone, reflexes, and reactions
- Section 2: Developmental milestones (head control, sitting, voluntary grasp, ability to kick, rolling, crawling, standing, and walking)
- Section 3: Behavioural assessment (state of consciousness, emotional state, social orientation)
HINE Section 2 (motor milestones) includes 8 items scored on a 5-point scale with 0 as the absence of activity, and a maximum score of 4 points7
- Some items have a maximum score of 2 or 3 points (see table below)
HINE Section 2 scoring chart illustrating the motor
developmental milestones7
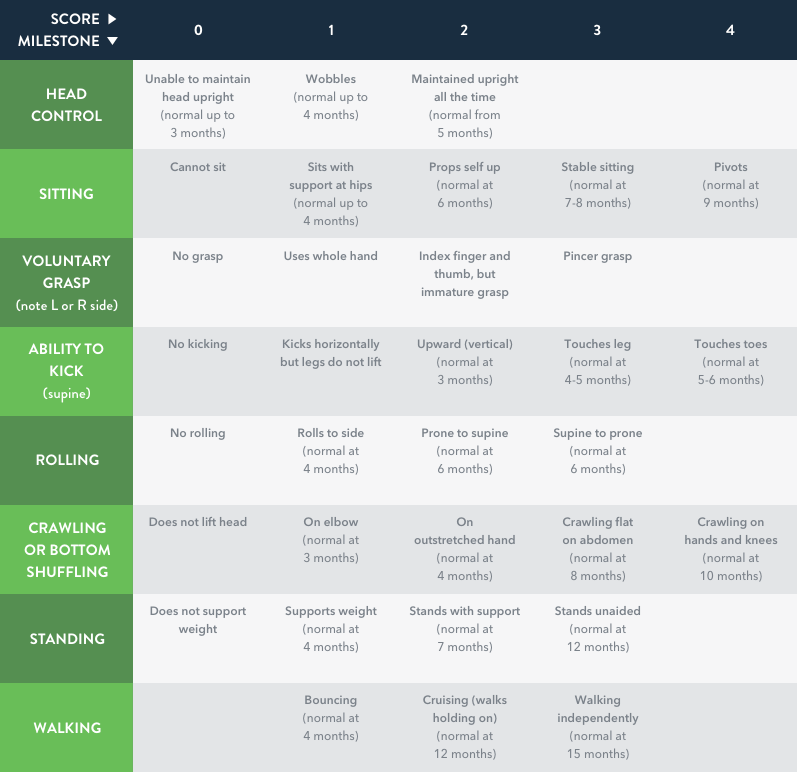
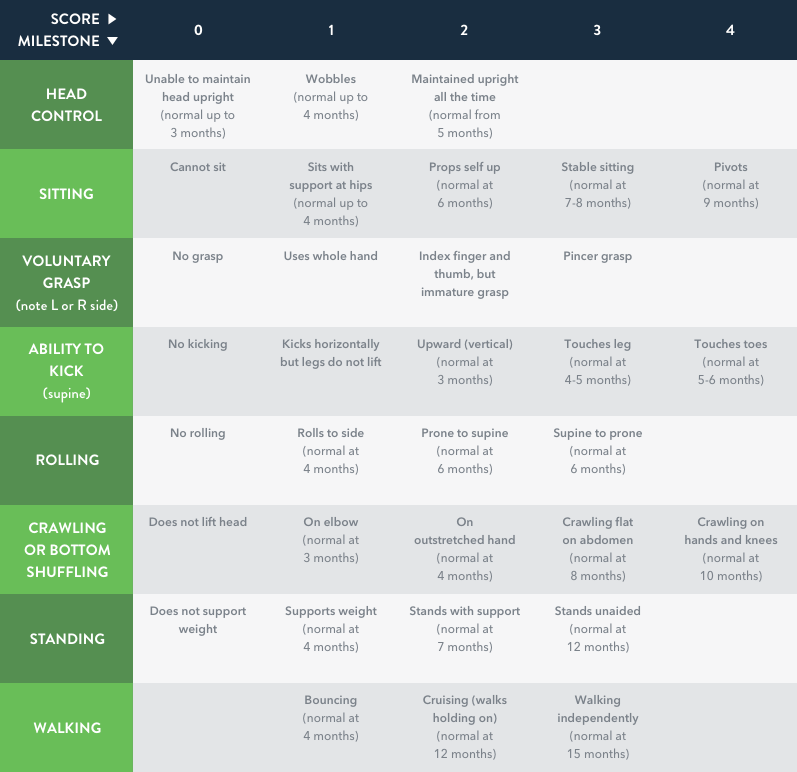
Adapted from DeSanctis et al, 2016.7
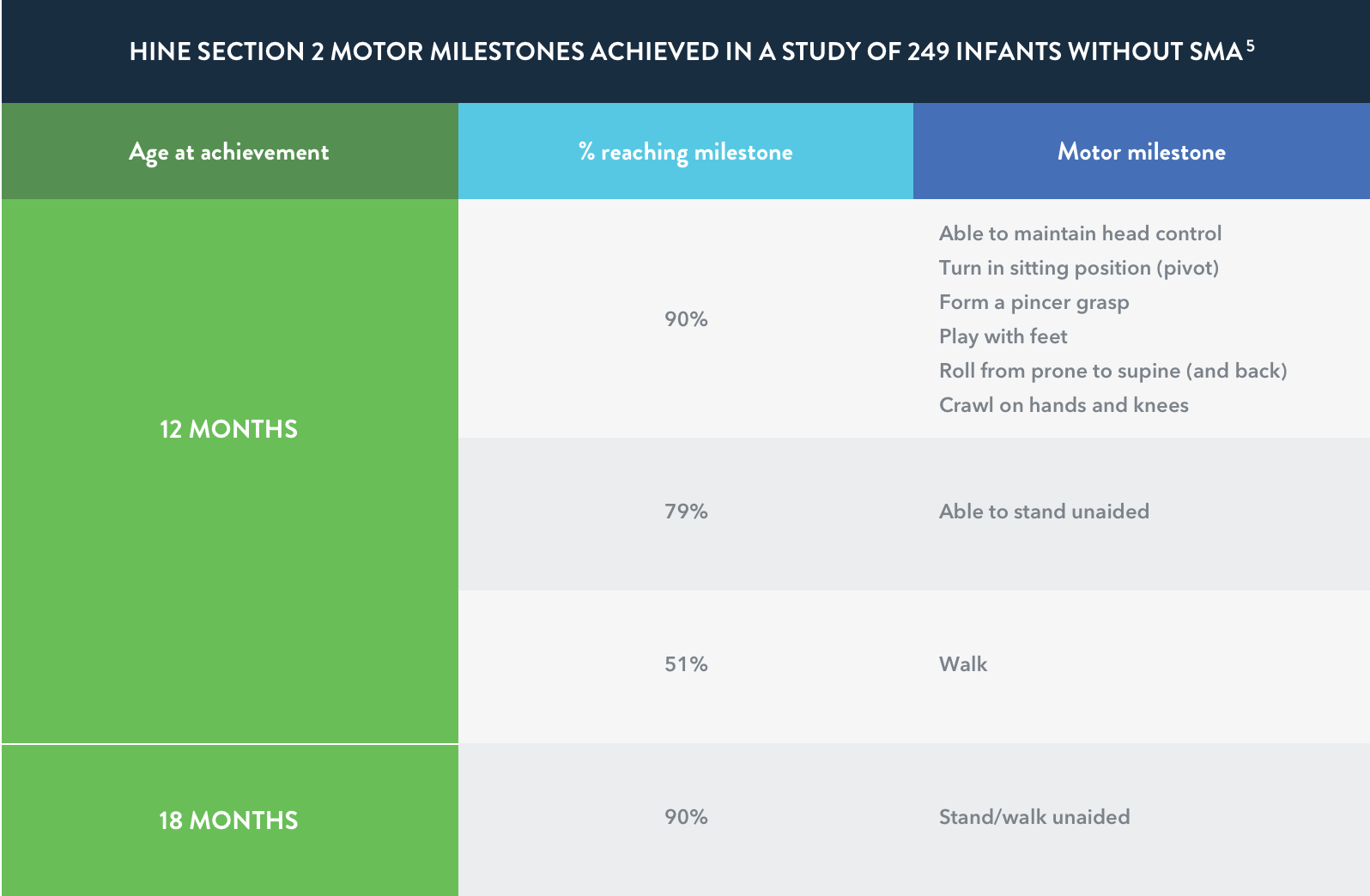
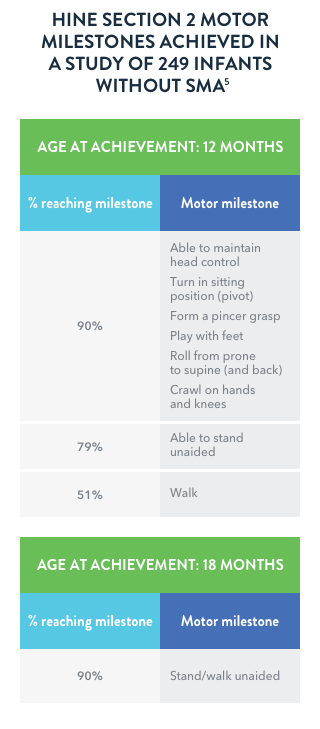
Motor milestone achievements are rare in
infantile-onset (Type I) SMA
In a retrospective study of individuals (n=33) with infantile-onset (Type I) SMA who were 1 to 8 months of age at the onset of symptoms, none of the more severely affected infants achieved a major milestone such as rolling over, independent sitting, crawling, standing, or walking.
Motor milestones are rarely acquired in infantile-onset SMA. Infants with the most severe symptoms of SMA (early onset) may show a score of 0 on all 8 items of the HINE Section 2.7
The Children’s Hospital of Philadelphia Infant Test of
Neuromuscular Disorders (CHOP INTEND)8,9
- CHOP INTEND was developed by evaluating infants (n=26) with Type I SMA, mean age 11.5 months (1.4-37.9 months), and has been shown to be valid for the assessment of children ranging in age from 3.8 months to over 4 years
- Includes 16 items used to assess motor skills.
Each item is graded on a scale of 0-4:10
0=No response
4=Complete response - Total score ranges from 0-64
Infants with SMA may score significantly lower on
CHOP INTEND than unaffected infants
Kolb et al. conducted a prospective, longitudinal natural history study of infants with genetically confirmed SMA that compared their CHOP INTEND scores with those of healthy infants. Age of onset of SMA symptoms ranged from <1 month to
4 to 5 months.4
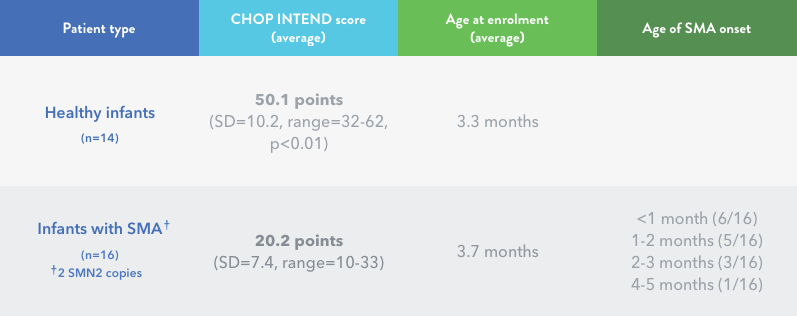

A CHOP INTEND score >40 is rarely observed for symptomatic individuals with infantile-onset (Type I) SMA who have 2 SMN2 gene copies.11
The Hammersmith Functional
Motor Scale—Expanded (HFMSE)
HFMSE is a measure that has been used in several clinical trials to evaluate the motor function of individuals with later-onset (Type II and Type III) SMA
The HFMSE includes 13 clinically relevant items from the Gross Motor Function Measure (GMFM) related to lying/rolling, crawling, crawling/kneeling, standing, and walking/running/jumping:3,12,13
- Exam has 33 items that are scored on a scale of 0–2
- Total score ranges from 0 to 66, with lower scores indicating poorer motor function
- Patient fatigue is an important consideration; the HFMSE can be conducted in 12 minutes (mean time)
Individuals with later-onset (Type II and Type III) SMA
may demonstrate progressive decline in HFMSE scores3
In one natural history study of SMA, individuals with later-onset SMA declined by 0.56 points (mean) in HFMSE score over 12 months.3
However, in another study of individuals (n=79) with later-onset SMA, motor function appeared to decline in a nonlinear fashion. The mean change in HFMSE scores at 36 months was -1.71 (p=0.01). During the study:14
- 2 patients with 2 copies of the SMN2 gene lost the ability to sit
- 1 patient with 3 copies of the SMN2 gene lost the ability to sit
- 5 patients with 3 copies of the SMN2 gene lost the ability to walk
The Upper Limb Module (ULM)
The ULM was developed to assess aspects of function related to everyday life in nonambulatory individuals with SMA. These skills might only be partly captured by the HFMSE in weaker patients.15
The ULM has been validated in the assessment of individuals with SMA (aged 30 months-27 years), including nonambulatory young and weaker children.16
The module includes 9 tasks that can be performed in a brief amount of time (5-10 minutes) using common equipment (e.g., drawing a continuous line with a pencil, picking up a coin and placing in a cup, pressing a button to turn on a lamp, lifting a beverage can to drink, removing the lid from a plastic container, lifting a weight and moving it from circle to circle on pre-printed paper). The maximum score possible is 18.16
ULM scores may remain relatively stable over
a 12-month period.15
A study was conducted with nonambulatory individuals (n=74) with later-onset SMA (Type II and Type III); age range was 3.5 to 29 years (mean 10.22, SD 6.15).15
The mean change in ULM at 12 months was 0.04 points (SD 1.17) from baseline (mean 10.23, SD 4.81). Most of the ≥2-point changes in ULM occurred in children who were <5 years of age.15
- 79.7% of the patients had ±1-point change
- 2.7% of the patients had >2-point gain in ULM (age <5 years)
- 2.7% of the patients had <2-point loss in ULM (age <5 years)
Changes greater than ±1-2 points in the ULM may be considered clinically relevant.15
Revised Upper Limb Module (RULM): The ULM was revised to address a ceiling effect and make the test useful in a wider population of individuals with SMA. The RULM consists of a total of 20 items for a maximum score of 37. Activities are graded from 0 to 2.17
The 6-Minute Walk Test (6MWT)
The 6MWT is an objective evaluation of exercise capacity that may be used to assess function in ambulatory individuals with later-onset SMA:18
- Participants are instructed to walk as fast as possible along a 25-metre course on a flat linoleum surface, turn around a marker cone, and return in the opposite direction18
- The loop is repeated as often as possible for 6 minutes18
- The test course has a start line, with horizontal lines placed every 1 metre. Running or jogging is not permitted18
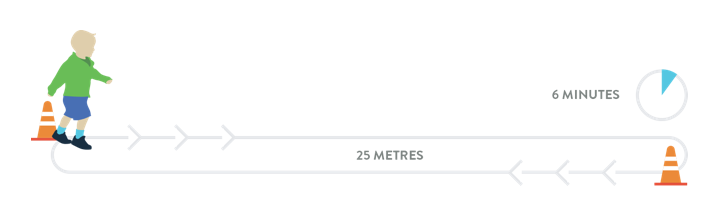
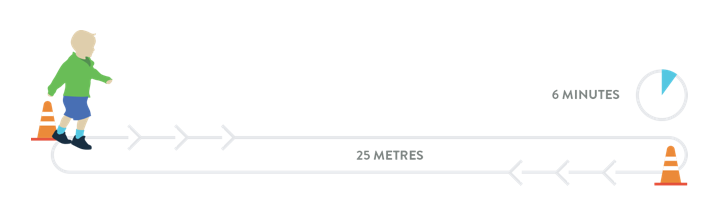
- In a study of ambulatory individuals (n=18) with later-onset SMA (4-48 years of age) the mean distance walked in 6 minutes was 289 metres18
- 6MWT distance is significantly associated with HFMSE score (r=0.83, p<0.0001)18
Progressive decline in 6MWT may occur in later-onset SMA.19
One study of ambulatory individuals with later-onset (Type III) SMA demonstrated a reduction of 1.5 metres per year from their baseline on the 6MWT.19
Electrophysiological measurements
Electrophysiologic measurements may be used to assess the health of motor neurons20
- Compound muscle action potential (CMAP) response is a measure of the electrophysiologic output from a specific muscle or muscle group following stimulation of the innervating nerve21
- Motor unit number estimation (MUNE) is a method that estimates the number of motor units‡ involved in the contraction of a specific muscle22
‡ Motor units include motor neurons and the muscle fibres they innervate.23
Trend lines represent CMAP declines in individuals with SMA. The shaded area indicates estimated normal values.
Adapted from Swoboda et al.20
In a clinical study, the average CMAP peak amplitude for infants with SMA was 1.4 mV (SD=2.2, n=25) compared with 5.5 mV in healthy infants (SD=2.0, n=27; p<0.01).4
Natural history studies among patients with Type I SMA demonstrate that CMAP amplitude is abnormally low and does not improve after symptom onset24
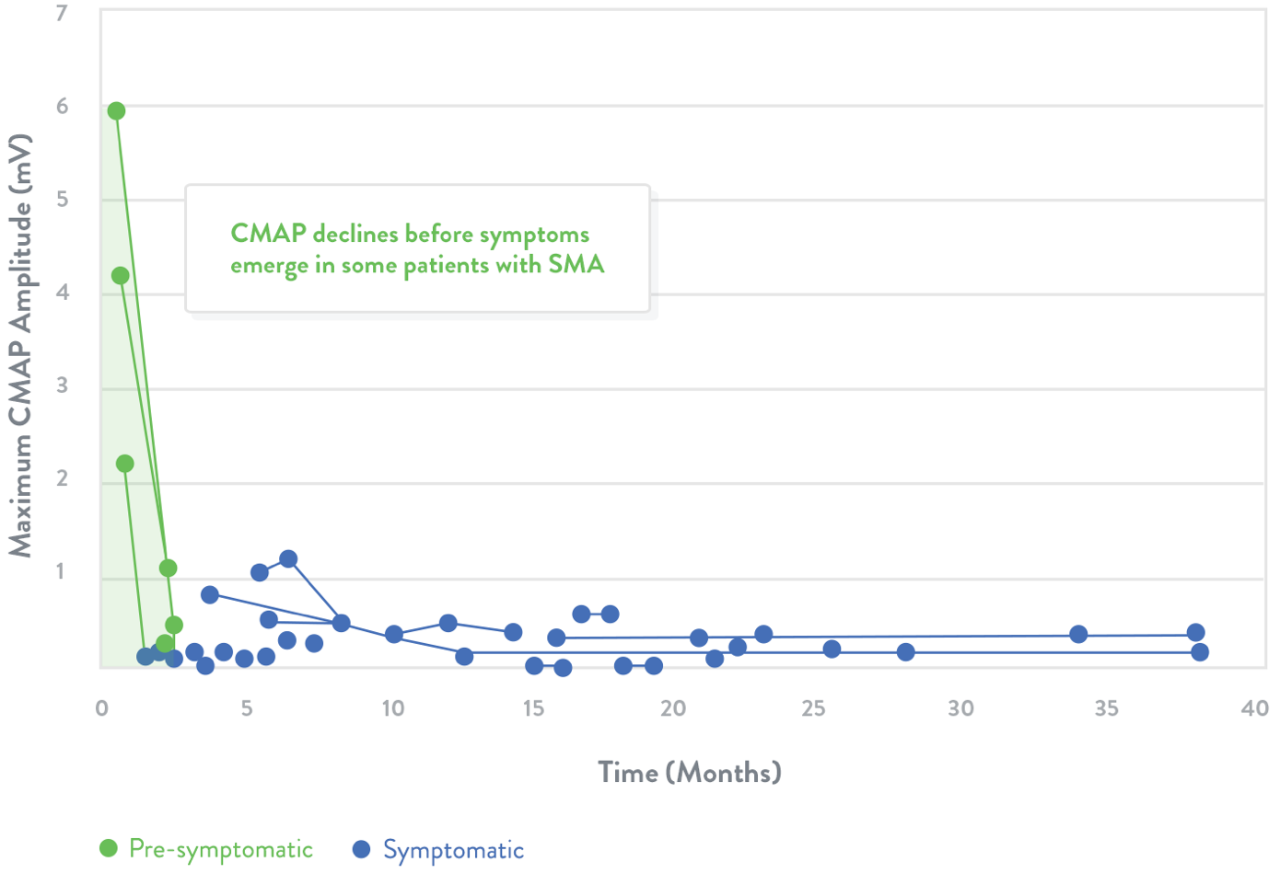
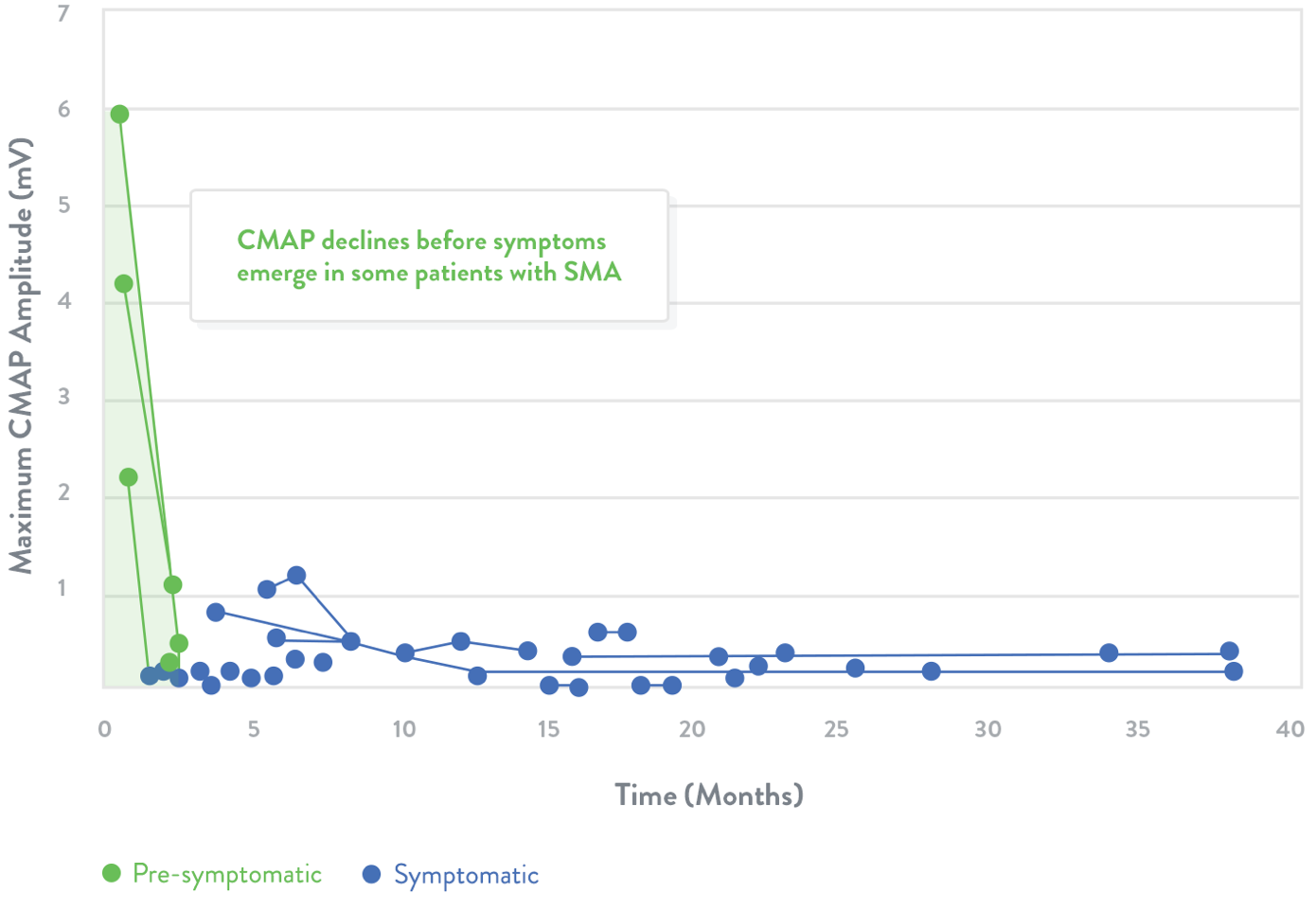
Green dots indicate children who were identified presymptomatically via genetic testing because a sibling was previously diagnosed with SMA.
Adapted from Swoboda et al.20
- Finkel RS et al. Neurology 2014; 83: 810–7.
- Darras BT et al. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician’s Approach. 2nd ed. London, UK: Elsevier; 2015.
- Mercuri E et al. Neuromuscul Disord 2016; 26: 123–31.
- Kolb SJ et al. Ann Clin Transl Neurol 2016;3:132–45.
- Haataja L et al. J Pediatr 1999; 135: 153–61.
- Romeo DM et al. Dev Med Child Neurol 2016; 58: 240–5.
- De Sanctis R et al. Neuromuscul Disord 2016; 26: 754–9.
- Glanzman AM et al. Neuromuscul Disord 2010; 20: 155–61.
- Glanzman AM et al. Pediatr Phys Ther 2011; 23: 322–6.
- Spinal Muscular Atrophy Clinical Research Center. CHOP INTEND for SMA Type I score sheet. Available at: http://columbiasma.org/links.html. Accessed November 2017.
- Finkel RS et al. Lancet 2016; 388: 3017–26.
- Glanzman AM et al. J Child Neurol 2011; 26: 1499–507.
- The Pediatric Neuromuscular Clinical Research Network for SMA. Expanded Hammersmith Functional Motor Scale for SMA (HFMSE). Available at: http://columbiasma.org/links.html. Accessed November 2017.
- Kaufmann P et al. Neurology 2012; 79: 1889–97.
- Sivo S et al. Neuromuscul Disord 2015; 25: 212–5.
- Mazzone E et al. Neuromuscul Disord 2011; 21: 406–12.
- Mazzone ES et al. Muscle Nerve 2017; 55: 869–74.
- Montes J et al. Neurology 2010; 74: 833–8.
- Mazzone E et al. Neuromuscul Disord 2013; 23: 624–8.
- Swoboda KJ et al. Ann Neurol 2005; 57: 704–12.
- Arnold WD et al. J Vis Exp 2015; 103: 1-8.
- Bromberg MB and Swoboda KJ. Muscle Nerve 2002; 25: 445–7.
- Monti RJ et al. Muscle Nerve 2001; 24: 848–66.
- Finkel RS. Neuromuscul Disord 2013; 23: 112–5.
